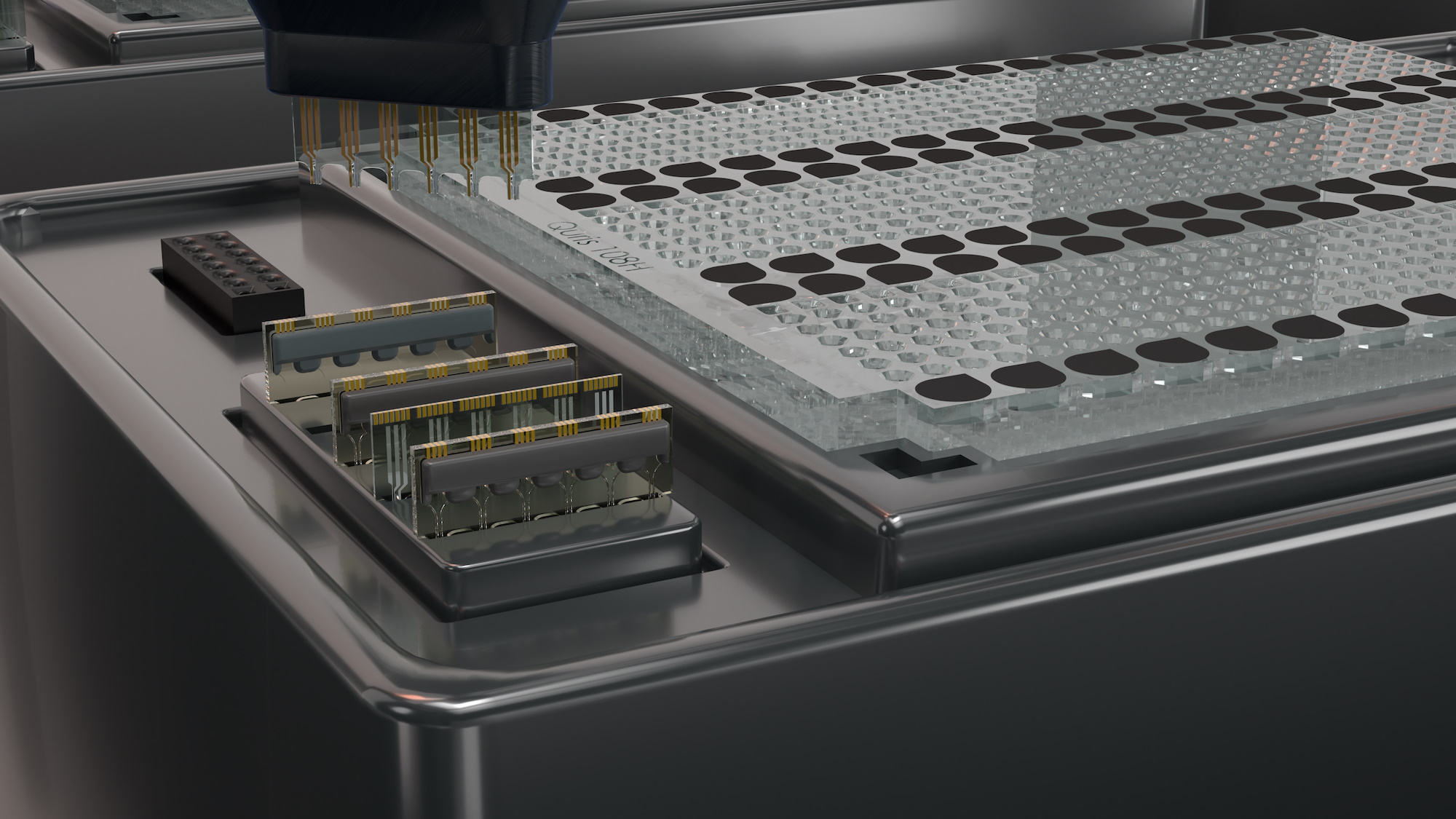
The necessity of animal testing is a sad one for the process of drug discovery, but there’s seemingly no good alternative to mice, even though they’re not particularly accurate human analogues. Quris claims to have the first real option in its combination of AI with data from a “patient on a chip” that provides remarkably robust testing and automation, all at a considerably lower cost — no mouse required.
The company has raised a $9M seed round to go from pilot to production, and an all-star team of backers and advisors are a promising indicator that the approach has fundamental merit.
The basic idea makes perfect sense: build a better small-scale simulation of a human body and use it to gather data that a machine learning system can easily interpret. It’s easier said than done, of course, but no sooner did researchers say it than Quris started doing it.
The Israel-based company’s approach builds on a major study at Harvard concerning the use of so-called “organs on a chip.” These systems, still relatively new but established in the field, use a small amount of stem cell-derived tissue (“organoids”) as a test bed for drugs or treatments — providing a good idea of how, for example, a human liver might respond to a combination of substances.
What the Harvard researchers found is that by linking multiple organ-on-a-chip systems together (like liver to kidney to heart cells), you end up with a surprisingly effective simulation of the human body. There’s nothing like the real thing, of course, but this serial organoid system or “patient on a chip” could be a real alternative to mouse testing; that’s still the most common way of seeing how a treatment affects a complete organ system, despite the fact that substances that pass the mouse phase only succeed in human tests about ten percent of the time.
CEO and co-founder Isaac Bentwich said that as soon as that study came out, he and his colleagues recognized the potential and started working on what needed to be done in terms of engineering and AI in order to turn this from an experimental system to a scalable product. It’s not just a mouse replacement, either — it’s a (relatively) inexpensive method to do limited human testing without the humans, and without the uncertainty of mice.
“Say you’re a pharma company,” said Bentwich in an interview. “Do you want to wait until you’re at the brink of going to clinical testing to find out whether a molecule that looks good on paper is actually effective? You can make all the genomics discoveries you want, but it won’t get you past mice experiments, where it fails 90 percent of the time. This lets you pick the winning horse before you go to the race.”
Considering drug candidates can cost hundreds of millions to get to the clinical stage, it’s more than worth spending even a small fortune (think tens of millions) to weed out a few destined for failure. If the technique is accurate — and indications are that it is — then the risk is practically nil and it will pay for itself if even a single expensive dead end is avoided. In other words, Bentwich put it, this brings the “fail early, fail cheap” mentality of software to a domain where neither was really an option.
The Quris system uses what it calls a chip-on-chip technique, in other words multiple organoid systems (chips) in sequence (on another chip), but smaller and more efficient than state of the art lab systems by an order of magnitude. It would cost millions to run a hundred simulated humans the way Harvard’s researchers did it, but thousands to do so on Quris’s system, which uses less raw biological material, can be automated, and is accompanied by a well-trained machine learning model.
That’s the other aspect that Quris is playing up: that this unique set of data will drive a unique AI that understands and can help run and interpret the experiments. The AI is already being trained with existing and some upcoming drugs, learning what signals from the various sensors mean for the safety of the substance. That allows effective testing to be done with a handful of chips rather than, say, 500 mice.
The chips themselves aren’t all the same, either. By manipulating and selecting the stem cells and tissues carefully, different types of people and different conditions or phenotypes can be tested for. If a company has a drug that works well but causes side effects 10 percent of the time and they don’t know why, testing against different genetic predispositions or complicating factors in an automated setting may be able to find out what genetic factors lead to those side effects.
Since the AI is aware of and cataloguing all this, it should get quite good at telling from a relatively small number of automated tests (think dozens, not thousands, and at a cost of thousands, not millions) whether a drug is a good candidate for human testing or not. Without the AI interpreting it, the data suddenly becomes a multi-PhD type of problem. But Bentwich was quick to note that they in no way anticipate eliminating the biological side and relying only on the AI. “It’s part of our philosophical and biological understanding that the AI has to work with a biological counterpart,” he said.
Robert Langer, co-founder of Moderna, is on the scientific advisory board, and told TechCrunch in the same interview that he concurred, and expects this technique to be adopted quickly, but not necessarily by the naturally conservative larger pharma companies.
“This does seem to be a very big opportunity,” he said. “I’ve had similar ideas in other areas of chemistry, that you can use AI to make these predictions. It certainly won’t replace testing but it narrows the possibilities, and my view is it would speed things up tremendously.”
It’s nice to have someone like Langer on your side (along with Nobel laureate Aaron Ciechanover), but Bentwich said that they’re relying more on their patent portfolio and first mover advantage to get a foot in the door. An agreement with the NY Stem Cell Foundation gives them special access to that organization’s stem cell workflow.
There are two prongs to the business model. One is providing the service to a pharma company of screening their drug candidates, with payment contingent on the results proving to be accurate — for example, a drug cleared by the system makes it to a given testing milestone as predicted. The other is to work on their own drugs; right now the company has a treatment for the Autism-linked Fragile X condition that it will be taking to clinical testing next year.
Bentwich pointed out that despite the proliferation of and investment in AI-powered drug discovery, few companies can claim to have a molecule resulting from their work entering clinical trials. This isn’t because they aren’t doing what they claim, for instance identifying molecules with certain bioreactivity or a method to manufacture them efficiently, but rather because there are so many other steps in the long discovery, testing, and approval process that the chance of making it through, while higher than it once was, is still very low.
The $9M seed round “funds us very well to finish the productization of the setup that we have, making it more efficient and automated, and testing the first hundred or thousand initial drugs to train the AI,” Bentwich said. The round was led by “Dr. Judith Richter and Dr. Kobi Richter, pioneers of cardiovascular intervention therapeutics, with participation from Moshe Yanai, a disruptive data-storage technology leader, and strategic angel investors,” as the press release puts it. A distinct lack of institutional capital — draw your own conclusions there.
Bentwich’s vision for the company is part of his general vision of “completely personalized medicine.” If the cost of stem cells continues to drop (already it has gone from millions to thousands), it would open up entirely new markets.
“It’ll no longer be just doing expensive experiments for pharma companies. In 5-10 years time this may well be what hundreds of millions of people are doing. If you think about it, it’s actually kind of barbaric how we’re living now,” he said. “You go to the pharmacist and they list the possible side effects, but you don’t know for sure. What, are you the guinea pig? The answer is: yes, we’re all guinea pigs. But this is a first step away from that.”
via https://AiUpNow.com October 18, 2021 at 07:35AM by Devin Coldewey, Khareem Sudlow,